For those starting clinical research
J-SUPPORT is actively involved in developing the next generation of researchers who are expected to revitalize clinical research in the field of cancer supportive care and disseminate research results.
People venturing into research for the first time and people wanting brush up their knowledge on research should use the useful tips on clinical research.
Please refer to the Development Strategy Map if you would like to know about clinical issues in typical fields of research on cancer supportive care in Japan.
J-SUPPORT also regularly holds seminars and workshops.
Seminars provide information on points to note when planning clinical research.
The workshops deliver lectures and also provide the opportunity for interactive learning, small group exercises, and simulated peer reviews.
Information on these events is posted on this website, on our Facebook page, and sent out to our mailing list. Please register as a J-SUPPORT member.
Information on events such as seminars and workshops
J-SUPPORT member registration (free of charge)
Research consultation
After registering as a J-SUPPORT member, you can apply for a research consultation, during which you can consult about your research with a mentor who has extensive experience in clinical research.
If the research is approved as J-SUPPORT-approved research after consultation, a mentorship system will be set up to provide careful mentoring, starting with advice on study design and statistical support for preparing study protocols, and ding a data management system, through to research management support, presentation, and paper preparationbuil.
J-SUPPORT member registration (free of charge)
Applying for research consultation
Research review
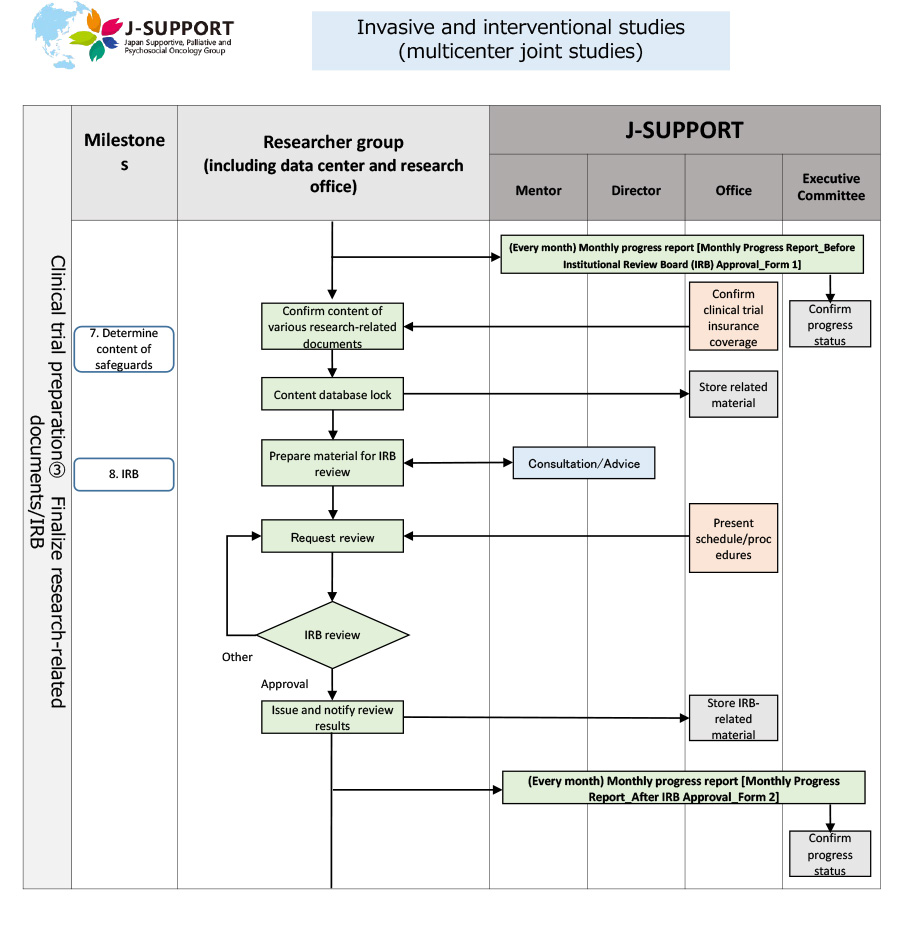
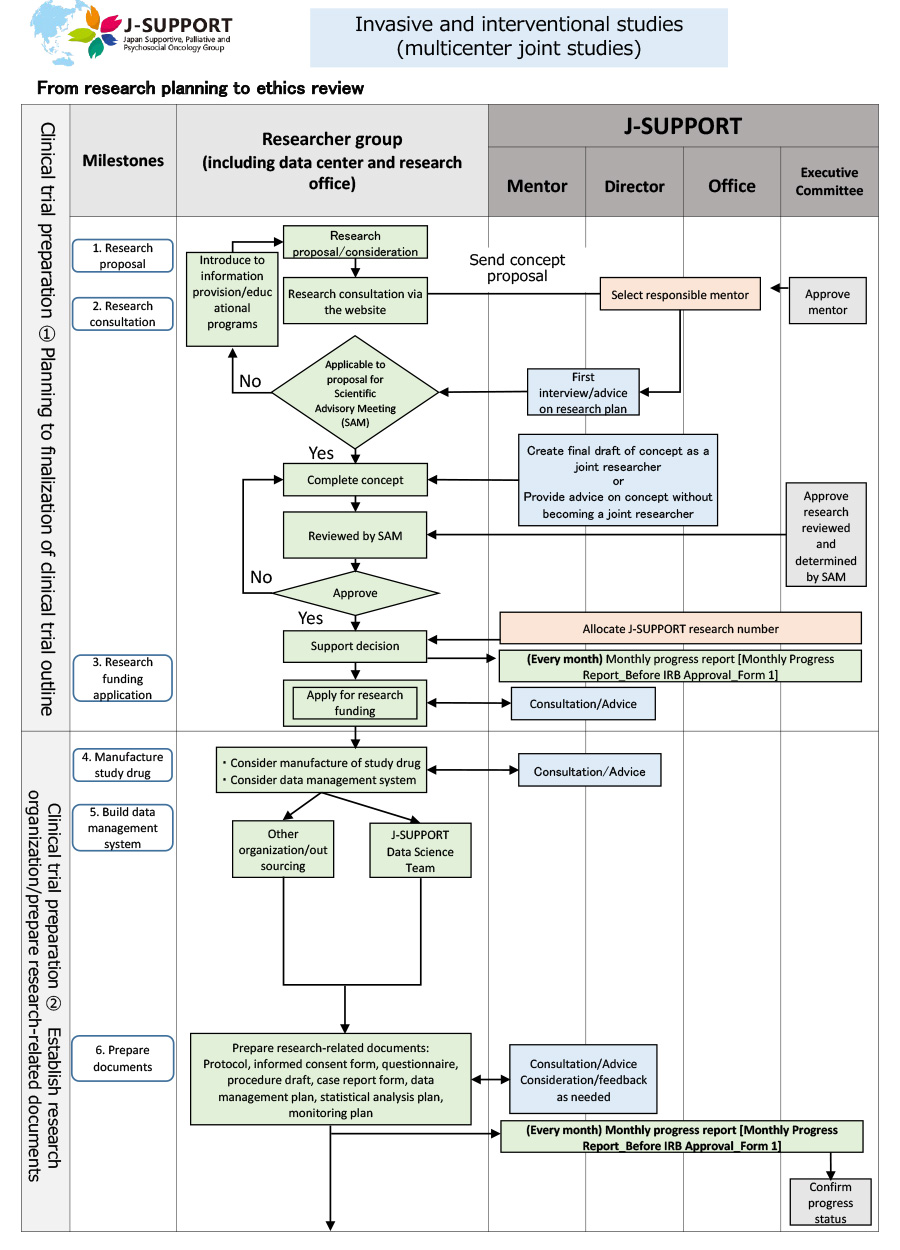
J-SUPPORT has introduced a protocol review committee (PRC) system that ensures the social validity of the study is reviewed by members of other J-SUPPORT groups and external reviewers who are not medical professionals. The PRC holds scientific advisory meetings as review meetings that focus first on whether the study can be completed (feasibility), and then, whether the study will have any effect on actual clinical practice after the study is completed (exit strategy). The review process not only scrutinizes the study but also provides advice on how to refine the study, as well as endeavors to encourage active participation of young researchers.
J-SUPPORT research road maps
This is an example of a research road map used when research is supported by J-SUPPORT. (Click to enlarge the image)
From research planning to ethics review of invasive and interventional studies (multicenter joint studies)
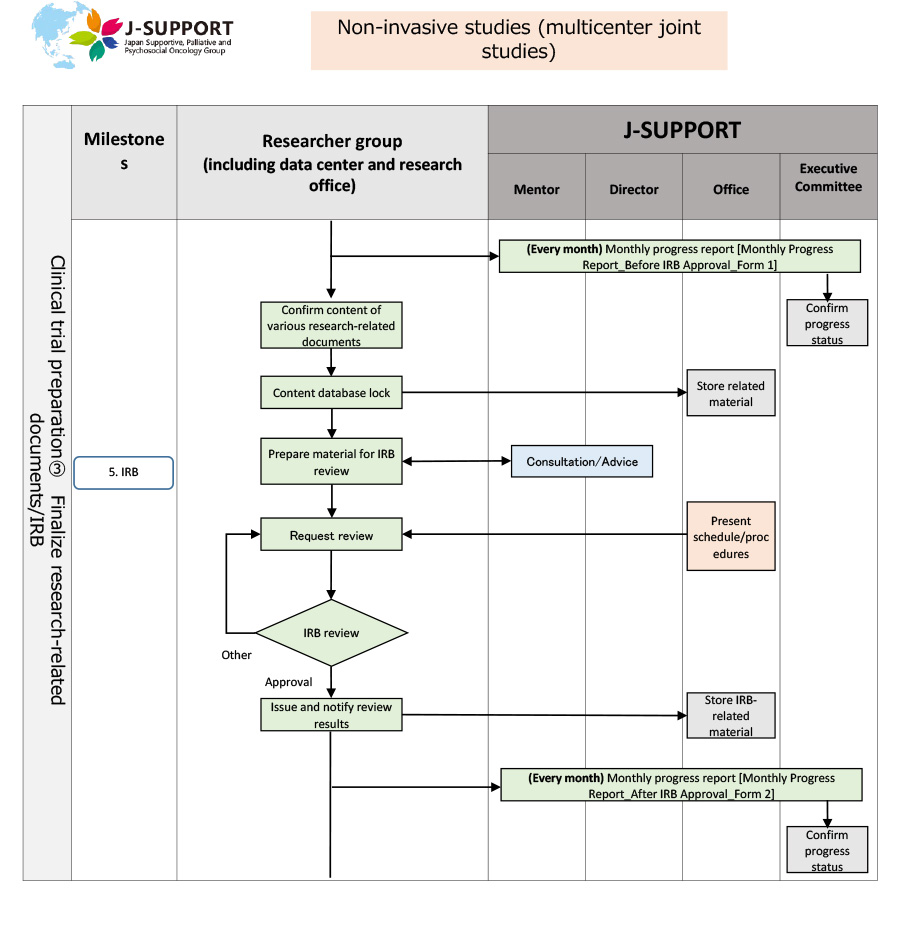
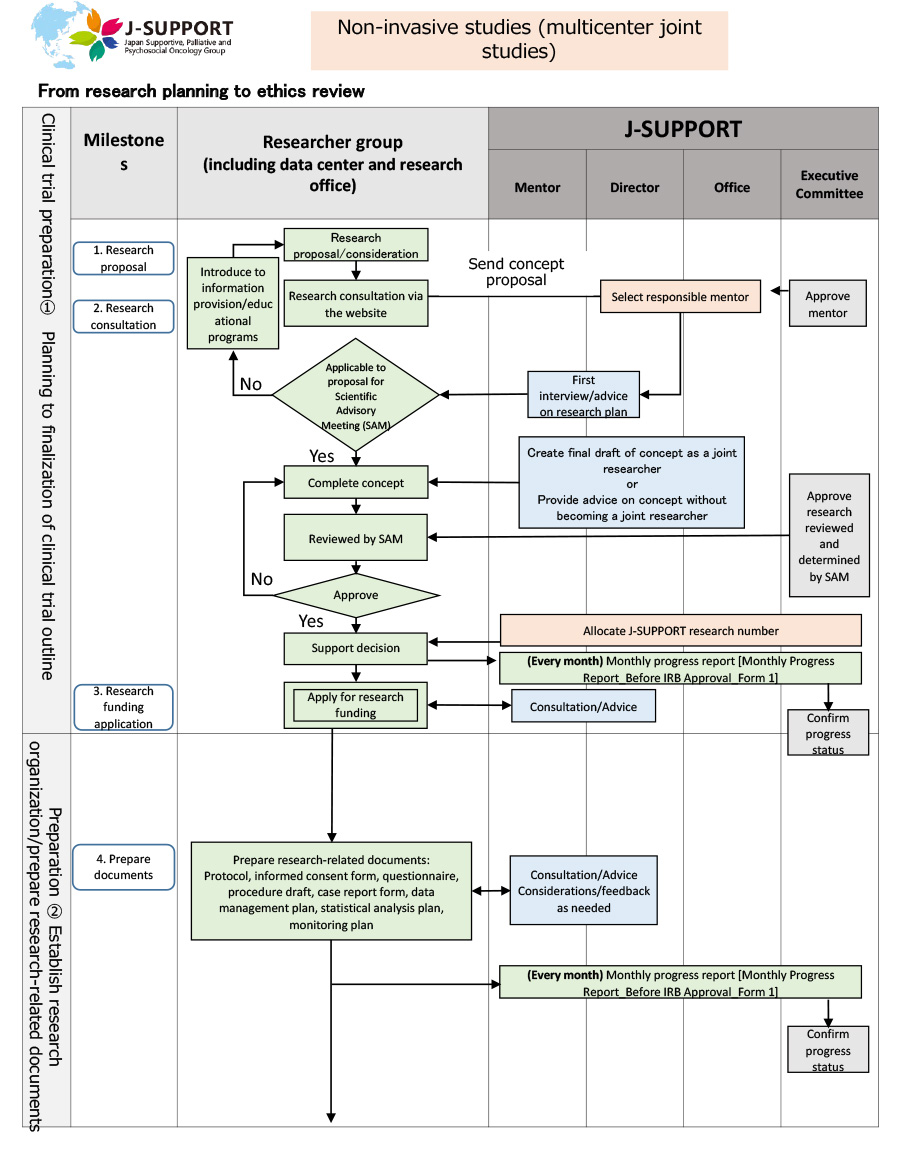
From research planning to ethics review of non-invasive studies (multicenter joint studies)
From the start of case registration to completion of the study (common to both invasive and non-invasive studies)
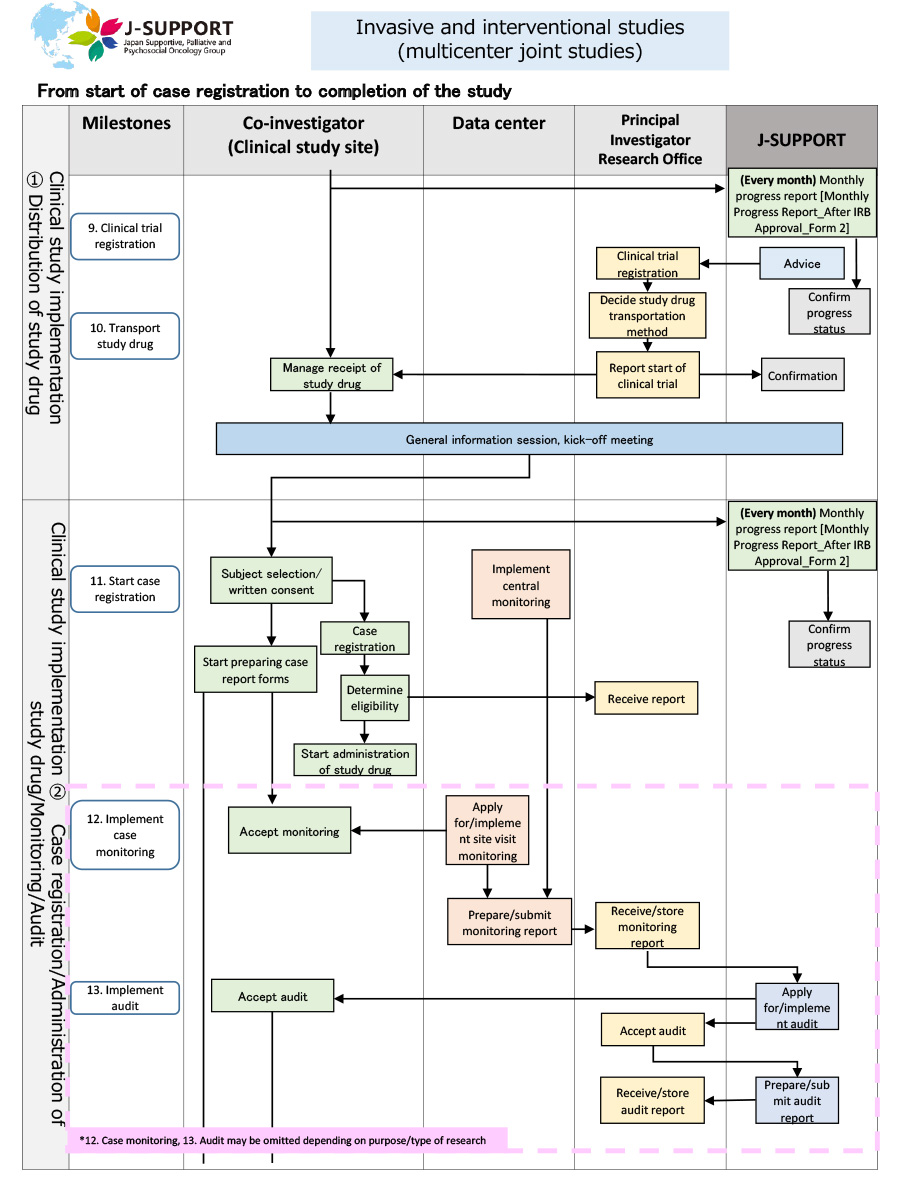
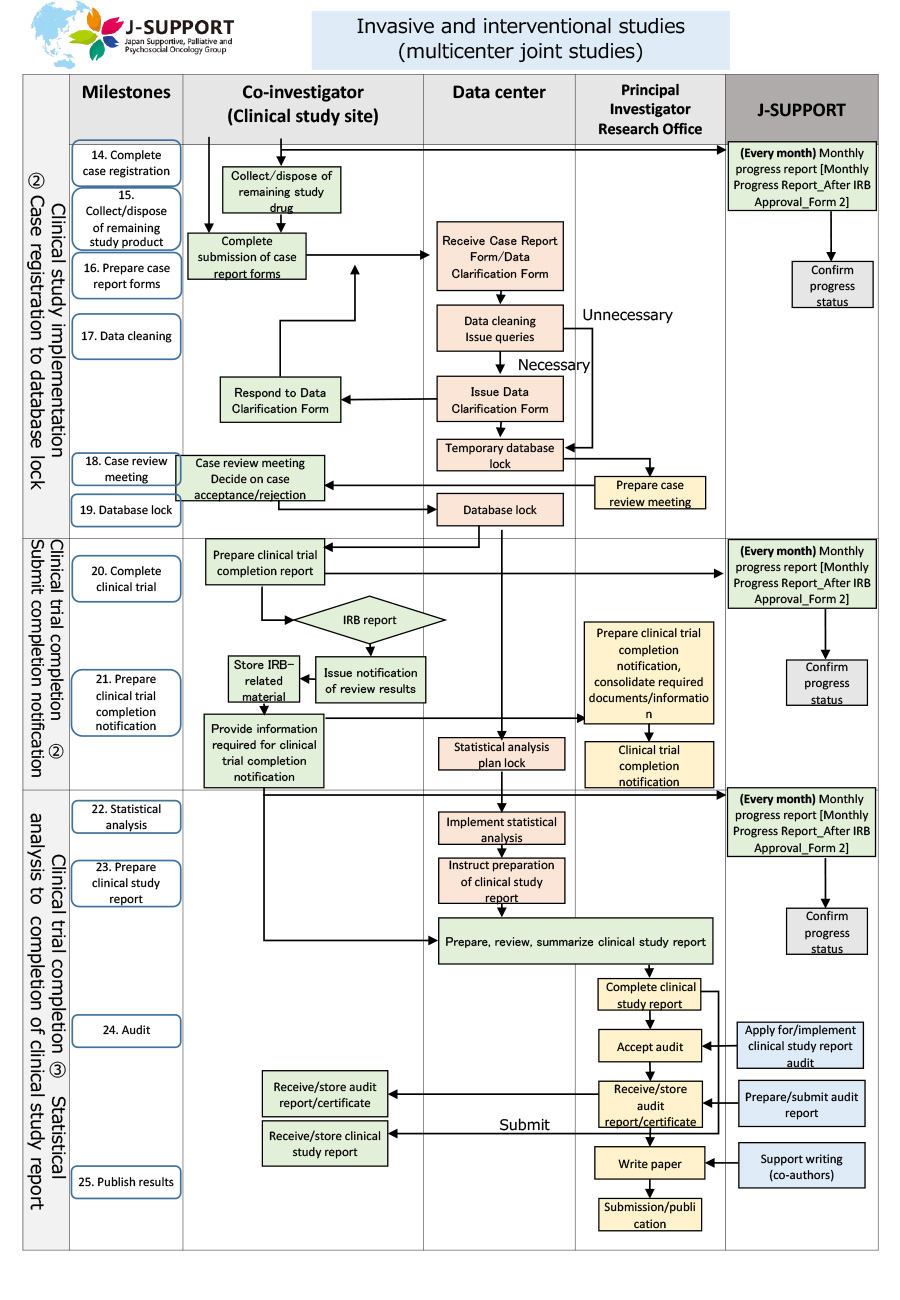
*The flow may change or may not apply depending on the purpose and content of the study.
*Requests when including the name J-SUPPORT on published documents
Consent must be obtained from the mentor and the management office when including the name J-SUPPORT on protocols and on applications for research funding. Please consult with your mentor after applying for research consultation.
1) Before approval by the scientific advisory meeting: Clearly state that it is under discussion with J-SUPPORT
2) After approval by the scientific advisory meeting: Clearly state the J-SUPPORT research number