J-SUPPORT1605
Yokukansan for perioperative psychiatric symptoms in cancer patients undergoing high invasive surgery: a randomized, double-blind, placebo-controlled trial
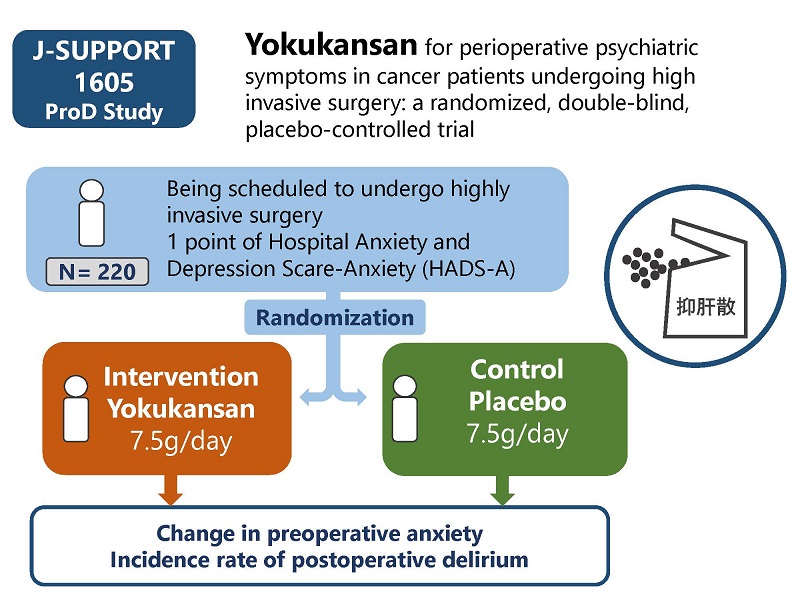
| Study Title | Yokukansan for perioperative psychiatric symptoms in cancer patients undergoing high invasive surgery: a randomized, double-blind, placebo-controlled trial |
|---|---|
| Principal Investigator/Study Manager/Mentor | Ken Shimizu/Saho Wada/Takuhiro Yamaguchi |
| Condition | Patients scheduled to undergo highly invasive surgery |
| UMIN Clinical Trials Registry | UMIN000027561 |
| Funding Source | Founded Study |
| Protocol Paper | Yokukansan for perioperative psychiatric symptoms in cancer patients undergoing high invasive surgery. J-SUPPORT 1605 (ProD Study): study protocol for a randomized controlled trial. Trials. 2019 Feb 8;20(1):110. doi: 10.1186/s13063-019-3202-1. PMID: 30736826 https://www.ncbi.nlm.nih.gov/pubmed/30736826 |
| Publication | Yokukansan for Treatment of Preoperative Anxiety and Prevention of Postoperative Delirium in Cancer Patients Undergoing Highly Invasive Surgery. J-SUPPORT 1605 (ProD Study): A Randomized, Double-Blind, Placebo-Controlled Trial. J Pain Symptom Manage. 2021 Jan;61(1):71-80. doi: 10.1016/j.jpainsymman.2020.07.009. Epub 2020 Aug 12. PMID: 32800969 https://pubmed.ncbi.nlm.nih.gov/32800969/ |
| Spreading effect |


