J-SUPPORT1602
TOPICal Steroid versus placebo for the prevention of radiation dermatitis in head and neck cancer patients receiving chemoradiotherapy : a phase III, randomized, double-blinded trial
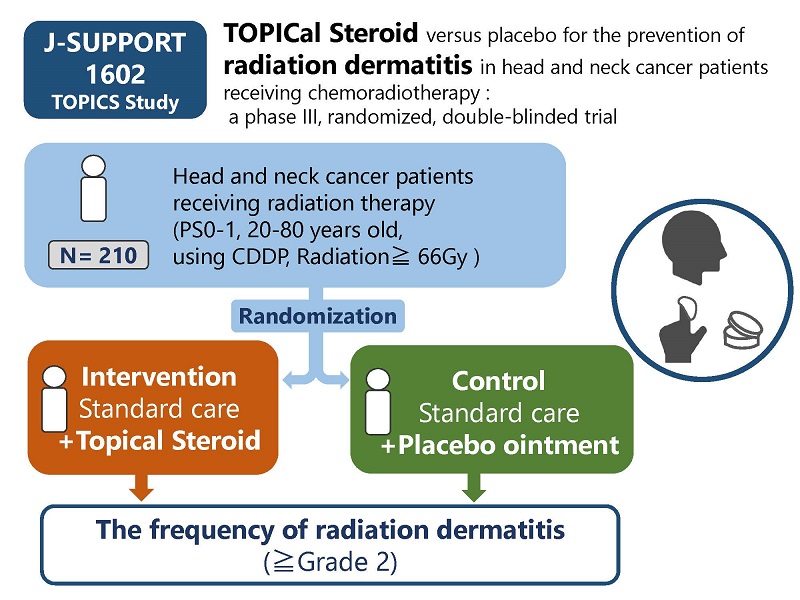
| Study Title | TOPICal Steroid versus placebo for the prevention of radiation dermatitis in head and neck cancer patients receiving chemoradiotherapy : a phase III, randomized, double-blinded trial |
|---|---|
| Principal Investigator/Study Manager/Mentor | Sadamoto Zenda/Tomoya Yokota/Takuhiro Yamaguchi |
| Condition | Locally Advanced Squamous Cell Carcinoma of the Head and Heck |
| UMIN Clinical Trials Registry | UMIN000027161 |
| Funding Source | The National Cancer Center Research and Development Fund (27-A-3), Private Foundation |
| Protocol Paper |
Topical steroid versus placebo for the prevention of radiation dermatitis in head and neck cancer patients receiving chemoradiotherapy: the study protocol of J-SUPPORT 1602 (TOPICS study), a randomized double-blinded phase 3 trial. BMC Cancer. 2018 Sep 6;18(1):873. doi: 10.1186/s12885-018-4763-1. PMID: 30189840 |
| Publication |
Phase 3 Randomized Trial of Topical Steroid Versus Placebo for Prevention of Radiation Dermatitis in Patients With Head and Neck Cancer Receiving Chemoradiation Int J Radiat Oncol Biol Phys. 2021 Nov 1;111(3):794-803. doi: 10.1016/j.ijrobp.2021.05.133. PMID: 34102298 |
| Spreading effect |


